Navigating Zinc
Why is zinc crucial not only for rust prevention but also for the next generation of batteries and clean energy storage?
Scientific Properties of Zinc
Zinc is a bluish-white metal with atomic number 30 and symbol Zn. It has a density of 7.14 g/cm³, a melting point of 419.5°C, and is commonly found as zinc sulfide (sphalerite) or zinc carbonate (smithsonite) in the Earth’s crust. Zinc is extracted from its ores through roasting and leaching processes and is known for its corrosion resistance, malleability, and electrical conductivity.
Uses and Future Applications
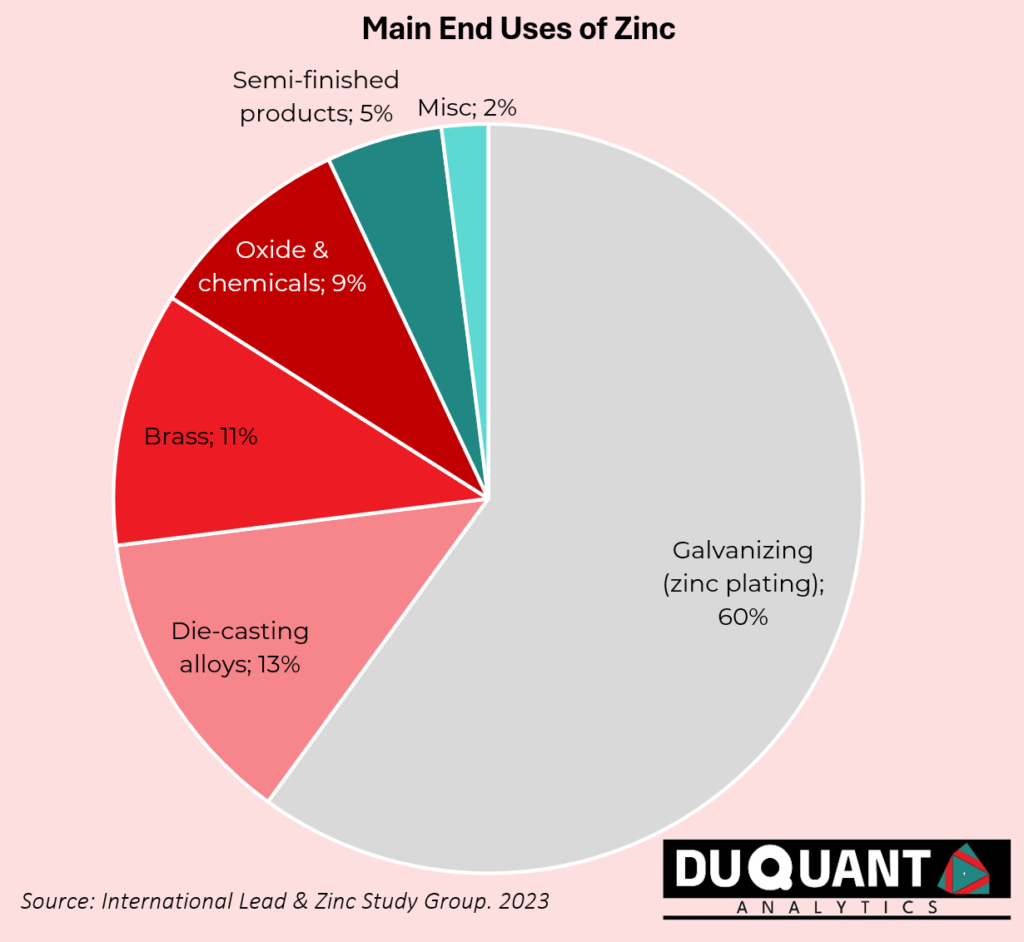
Zinc is an essential metal with a wide range of applications across various industries, and its future potential continues to expand with ongoing research and technological advancements. Zinc is commonly used for galvanizing steel and iron to prevent rust and corrosion. It coats these metals, extending their lifespan, particularly in construction like bridges and vehicles. Zinc compounds, like zinc oxide and zinc sulphate, are widely used in the production of rubber, ceramics, glass, and paints. Zinc oxide is a semiconductor with applications in optoelectronics, sensors, and transistors. It is being researched for use in transparent conductive films for displays, solar cells, and LEDs.
Zinc is a material that can be alloyed with different metals such as Copper or Aluminium. Zinc is alloyed with copper in order to make brass, which is used in musical instruments, plumbing, and architectural applications. Zinc-Aluminium alloys are used in die-casting for producing automotive parts, hardware, and consumer goods due to their strength and lightweight nature of the alloy. Zinc-based alloys are being explored for use in 3D printing, especially in industries where lightweight and corrosion-resistant materials are essential in industries such as aerospace and automotive industries.
Zinc-Carbon and Zinc-Air Batteries are commonly used in household appliances and hearing aids. Zinc is the anode material in these batteries. Zinc is gaining attention in the development of next-generation batteries, such as zinc-ion and zinc-air batteries, for energy storage. These batteries are being researched as a potential alternative to lithium-ion batteries because of their lower cost, safety, and abundance. They are particularly promising for grid-scale energy storage in renewable energy systems.
Zinc’s diverse properties make it a critical material for both traditional uses, such as galvanization and health supplements, and emerging technologies in renewable energy, biotechnology, and advanced manufacturing. Future applications could see zinc playing a vital role in sustainability efforts, healthcare innovations, and the development of safer and more efficient technologies.
Largest Sources and Producers of Zinc
One of the significant risks facing zinc mining companies is the market volatility in zinc spot prices. Over recent years, fluctuations in supply and demand have influenced zinc prices, leading to potential concerns about oversupply or shortages, which can cause prices to rise or fall abruptly.
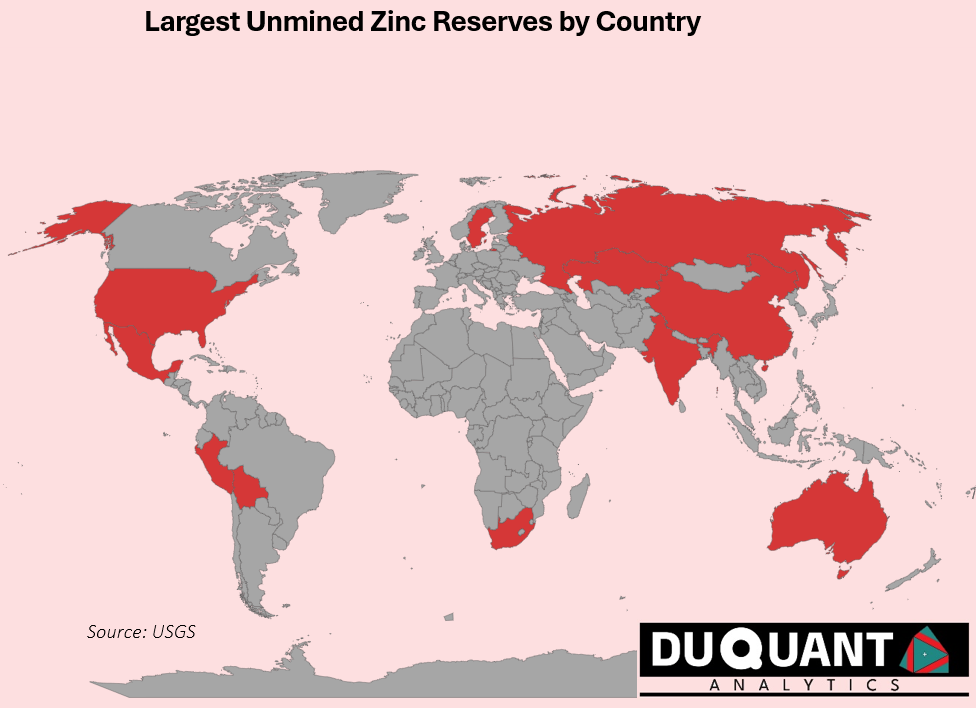
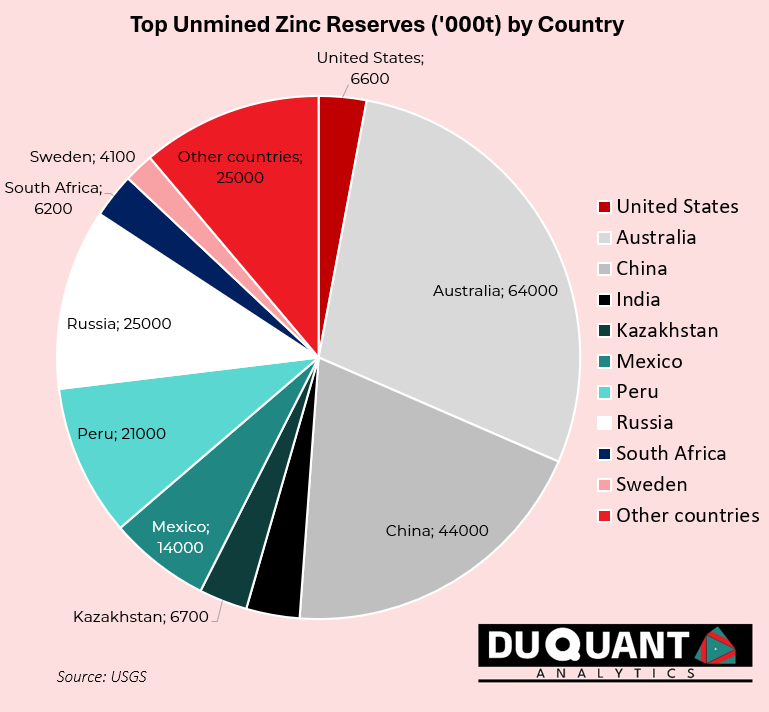
Zinc is found in many locations across the globe, and according to the 2024 US Geological Survey (USGS), global zinc reserves stand at approximately 250 million metric tons. The largest reserves are located in Australia (64 million metric tons), China (43 million metric tons), and Peru (23 million metric tons). The remainder of the world makes up around 120 million metric tons (or 48%).
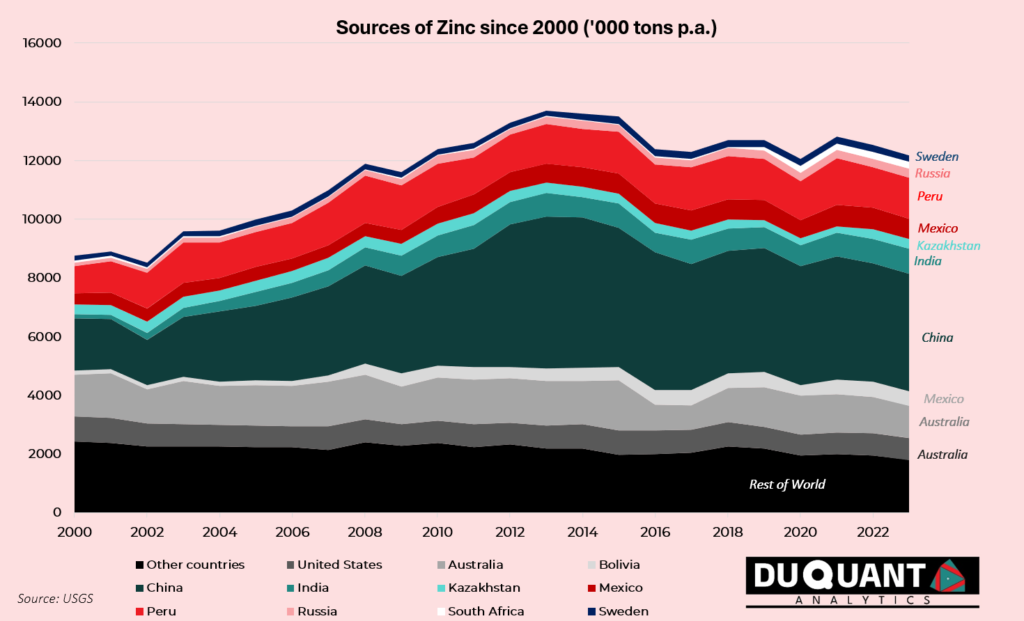
Zinc mine production has steadily declined in the recent decade, with global production rising from around 10 million metric tons in 2000 to 12 million metric tons by 2023. The majority of this increase can be attributed to China, which alone produces approximately 33% of the world’s zinc, followed by Peru (10%), Australia (10%), and India (7%). Other notable producers include the United States, Mexico, Bolivia, and Canada, contributing to the remaining global output.
In terms of global zinc reserves, Australia and China lead the world in zinc supply, ensuring future stability in production At the current rates of production (12,000Kt pa), the world has enough zinc to support mining operations for just over 18 years on existing reserves of 220,000Kt. Zinc prices remain volatile, and given less than 20 years of reserves remaining, impacted by market trends, supply chain disruptions, and fluctuations in demand. There is likely to be a growing need for new exploration activity for zinc around the world given its need.
The Remarkable History of Zinc
Zinc, a versatile metal crucial for modern industry, has a rich and varied history. From its early use in alloys to its critical role in contemporary technology, zinc’s journey is a fascinating tale of discovery, innovation, and transformation. This narrative highlight key events and discoveries that have shaped the zinc industry over the centuries.
The story of zinc begins in ancient times, long before the metal was isolated in its pure form. Early civilizations unknowingly used zinc in various alloys. Brass, an alloy of copper and zinc, was produced as early as 1,000 BCE in the Middle East and the Indian subcontinent. These early metallurgists discovered that adding zinc ore to molten copper resulted in a stronger and more malleable material, perfect for making tools, weapons, and decorative items. The Romans and Greeks also utilized brass extensively, though they did not understand the role of zinc in its production. They used this versatile alloy for coins, armour, and household items. The use of zinc compounds in medicine and cosmetics was also prevalent in ancient times, with zinc oxide being applied as an ointment for skin conditions.
The isolation of zinc as a distinct metal occurred much later. Indian metallurgists in the 13th century developed a method to extract zinc from its ores through a process of distillation. This technique was advanced for its time, involving the heating of zinc ore in a closed vessel and condensing the metal vapor. The resulting pure zinc was used to make brass and other items, marking a significant advancement in metallurgical practices. In Europe, the isolation of zinc remained elusive until the 17th century. Andreas Sigismund Marggraf, a German chemist, is credited with isolating pure zinc in 1746 through a similar distillation process. This discovery laid the groundwork for the industrial production of zinc and its widespread use in various applications.
The Industrial Revolution brought about a significant increase in the demand for zinc. The development of galvanization in the early 19th century, where iron or steel is coated with a layer of zinc to prevent rusting, revolutionized the construction and manufacturing industries. This process, discovered by Stanislas Sorel in 1837, highlighted zinc’s corrosion-resistant properties and significantly boosted its demand. During this period, major zinc deposits were discovered and mined in several regions. Notable discoveries included large deposits in the United States, Australia, and Europe. The expansion of railroads and infrastructure projects during the Industrial Revolution further increased the need for galvanized steel, cementing zinc’s importance in industrial applications.
The early 20th century saw significant advancements in zinc production techniques. Electrolytic refining, developed in the early 1900s, allowed for the production of high-purity zinc on an industrial scale. This method involved dissolving zinc ore in sulfuric acid and using electrolysis to extract the pure metal. The electrolytic process improved the efficiency and quality of zinc production, making it more accessible for various uses.
During both World Wars, zinc played a crucial role in military applications. It was used extensively in the production of brass cartridges, artillery shells, and other military equipment. The strategic importance of zinc led to increased mining and refining activities to ensure a steady supply for the war effort. The post-World War II era saw a boom in consumer products, many of which relied on zinc. The automotive industry, in particular, used large quantities of galvanized steel for car bodies, enhancing durability and corrosion resistance. Zinc oxide became a key ingredient in rubber manufacturing, improving the longevity and performance of tires.
The 1980s marked the emergence of zinc as a critical component in battery technology. Zinc-carbon and alkaline batteries, which use zinc as the anode material, became widely used in consumer electronics. These batteries offered a reliable and cost-effective power source for a wide range of devices. The environmental impact of zinc mining and processing has become a significant concern in recent decades. The extraction and smelting of zinc ore generate waste materials and emissions that can be harmful to the environment. To address these issues, the industry has increasingly focused on sustainable practices, such as recycling zinc-containing products and reducing emissions from production processes. Recycling zinc from scrap metal and other sources has become an important aspect of sustainable zinc production. Innovations in recycling technology have made it possible to recover high-purity zinc from end-of-life products, reducing the need for primary mining and minimizing environmental impact. The 21st century has seen zinc play an increasingly important role in renewable energy and environmental applications. Zinc-air batteries, known for their high energy density and environmental friendliness, have gained attention as potential energy storage solutions for renewable energy systems. Additionally, zinc oxide nanoparticles are used in various environmental applications, including water purification and air quality control.
As the world continues to prioritize sustainability and renewable energy, zinc’s importance is set to grow. Research and development efforts are focused on enhancing the performance of zinc-based batteries, improving recycling methods, and finding new applications for zinc in sustainable technologies.
From its early use in alloys and medicine to its central role in modern industry and technology, zinc’s journey is a testament to its versatility and enduring value. Its story reflects the dynamic interplay between natural resources and environmental technological advancements, shaping the future of our industrial and escapes.
Download This Post in PDF
RESEARCH DISCLOSURE
Purpose: DUQUANT Analytics provides research notes and newsletters for market insights, analysis, informational, educational, and research purposes only. We adhere to the CFA Institute’s Code of Ethics and Standards of Professional Conduct which includes maintaining the highest standards of integrity, transparency and professionalism in all research activities. We strive to ensure that our investment analysis and actions remain unbiased and focused on delivering accurate information based on available knowledge at the time of release. We follow industry accepted methodologies for conducting due diligence and conduct our analysis on a reasonable basis.
Investor Responsibility and Limitation of Liability: This content does not constitute a recommendation to buy or sell any securities. It is essential to understand the nature of our research, which is for educational and informative purposes. It is imperative to conduct your own due diligence and research prior to making any investment. DUQUANT Analytics is not liable for any losses incurred from using our research. Information released is believed to be reliable, but not guaranteed for accuracy or completeness. It is also important to note that past performance is not reflective of future performance.
Potential Conflict of Interest: Personal Investments: Members of DUQUANT Analytics may hold positions in some of the assets or securities mentioned in our research notes and newsletters. This includes equities, bonds, commodities and other financial instruments. Despite any personal investments, our research is conducted with the highest level of integrity, independence and objectivity. We strive to ensure that our analysis remains unbiased by focusing on accurate, public and provable information and secondary data as it is released or becomes public.
Conclusion: DUQUANT Analytics is committed to providing high-quality research and insights to our readers. However, it is essential for investors to take responsibility for their own investment decisions and to understand the inherent risks involve, especially in mining which carries more risk than traditional assets. We encourage you to consult with a qualified financial advisor and to perform thorough research before making any investments.
Thank you for reading this disclosure note. We value your trust and strive to maintain the highest standards of integrity in all our research activities.

